Clinicaltrials Gov Prs Login
Clinicaltrials Gov Prs Login - The modernized prs is now the primary website for protocol registration. The classic prs remains available for users who need to. The unique identification code given to each clinical study upon. After logging in, you will be directed to the new website. Welcome to your new prs beta home page the national library of medicine (nlm) has launched an effort to modernize clinicaltrials.gov. Login with your institutional email. Follow our step by step. There are two types of clinical studies: Welcome to the clinicaltrials.gov protocol registration and results system (prs). The modernized prs is now the primary website for protocol registration. Login with your institutional email. There are two types of clinical studies: The modernized prs is now the primary website for protocol registration. The modernized prs is now the primary website for protocol registration. Start with our step by step registration instructions and our outcome measures tip sheet. Welcome to your new prs beta home page the national library of medicine (nlm) has launched an effort to modernize clinicaltrials.gov. The unique identification code given to each clinical study upon. After logging in, you will. The classic prs remains available for users who need to. Most users will not apply for a prs account directly with clinicaltrials.gov, but will do so through the study sponsor organization’s prs administrator. There are two types of clinical studies: Welcome to your new prs beta home page the national library of medicine (nlm) has launched an effort to modernize clinicaltrials.gov. We will be continually delivering. After logging in, you will be directed to the new website. The unique identification code given to each clinical study upon. There are two types of clinical studies: Welcome to your new prs beta home page the national library of medicine (nlm) has launched an effort to modernize clinicaltrials.gov. We will be continually delivering. Most users will not apply for a prs account directly with clinicaltrials.gov, but will do so through the study sponsor organization’s prs administrator. The modernized prs is. Most users will not apply for a prs account directly with clinicaltrials.gov, but will do so through the study sponsor organization’s prs administrator. The modernized prs is now the primary website for protocol registration. There are two types of clinical studies: After logging in, you will. The unique identification code given to each clinical study upon. Welcome to your new prs beta home page the national library of medicine (nlm) has launched an effort to modernize clinicaltrials.gov. We will be continually delivering. There are two types of clinical studies: Follow our step by step. Interventional studies (also called clinical trials) and observational studies. The modernized prs is now the primary website for protocol registration. Start with our step by step registration instructions and our outcome measures tip sheet. Login with your institutional email. Interventional studies (also called clinical trials) and observational studies. After logging in, you will be directed to the new website. After logging in, you will be directed to the new website. Start with our step by step registration instructions and our outcome measures tip sheet. There are two types of clinical studies: Interventional studies (also called clinical trials) and observational studies. Follow our step by step. Follow our step by step. Login with your institutional email. The modernized prs is now the primary website for protocol registration. Interventional studies (also called clinical trials) and observational studies. After logging in, you will. Most users will not apply for a prs account directly with clinicaltrials.gov, but will do so through the study sponsor organization’s prs administrator. Login with your institutional email. The modernized prs is now the primary website for protocol registration. The classic prs remains available for users who need to. Interventional studies (also called clinical trials) and observational studies. The modernized prs is now the primary website for protocol registration. The modernized prs is now the primary website for protocol registration. Follow our step by step. Start with our step by step registration instructions and our outcome measures tip sheet. There are two types of clinical studies: Most users will not apply for a prs account directly with clinicaltrials.gov, but will do so through the study sponsor organization’s prs administrator. The unique identification code given to each clinical study upon. Start with our step by step registration instructions and our outcome measures tip sheet. Login with your institutional email. The modernized prs is now the primary website. Follow our step by step. We will be continually delivering. After logging in, you will be directed to the new website. After logging in, you will. Welcome to the clinicaltrials.gov protocol registration and results system (prs). The classic prs remains available for users who need to. The modernized prs is now the primary website for protocol registration. Start with our step by step registration instructions and our outcome measures tip sheet. The modernized prs is now the primary website for protocol registration. Most users will not apply for a prs account directly with clinicaltrials.gov, but will do so through the study sponsor organization’s prs administrator. The unique identification code given to each clinical study upon. Interventional studies (also called clinical trials) and observational studies.Clinicaltrials Gov Login
Clinicaltrials Gov Login
Clinicaltrials Gov Login
Clinicaltrials Gov Login
Clinicaltrials Gov Login
A brief Introduction on ClinicalTrials.gov (PRS) and EU Clinical Trials Register (EudraCT)
Clinicaltrials Gov Login
ClinicalTrials.gov and CTRP DF/HCC
Clinicaltrials Gov Login
Clinicaltrials Gov Login
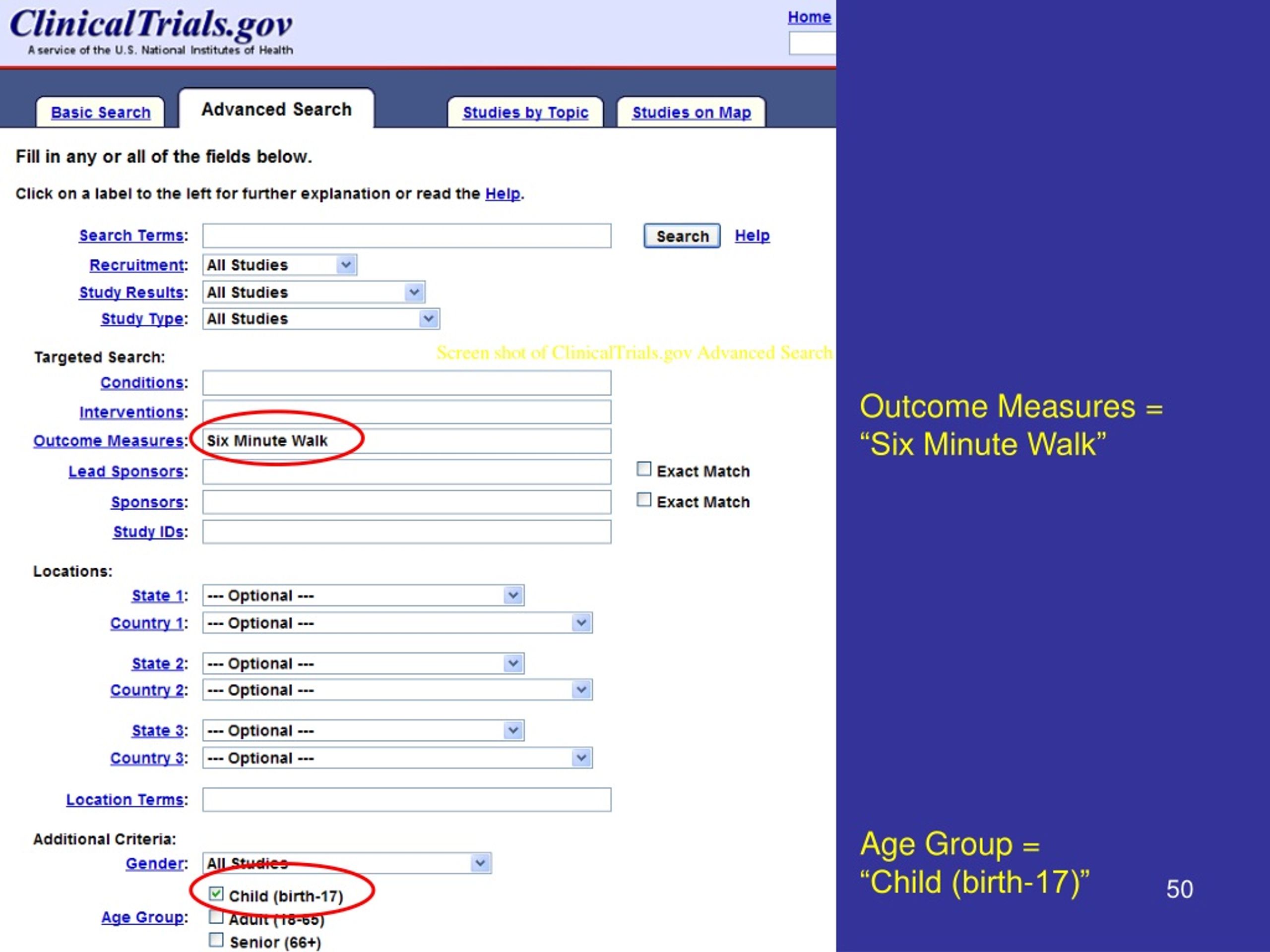
There Are Two Types Of Clinical Studies:
Welcome To Your New Prs Beta Home Page The National Library Of Medicine (Nlm) Has Launched An Effort To Modernize Clinicaltrials.gov.
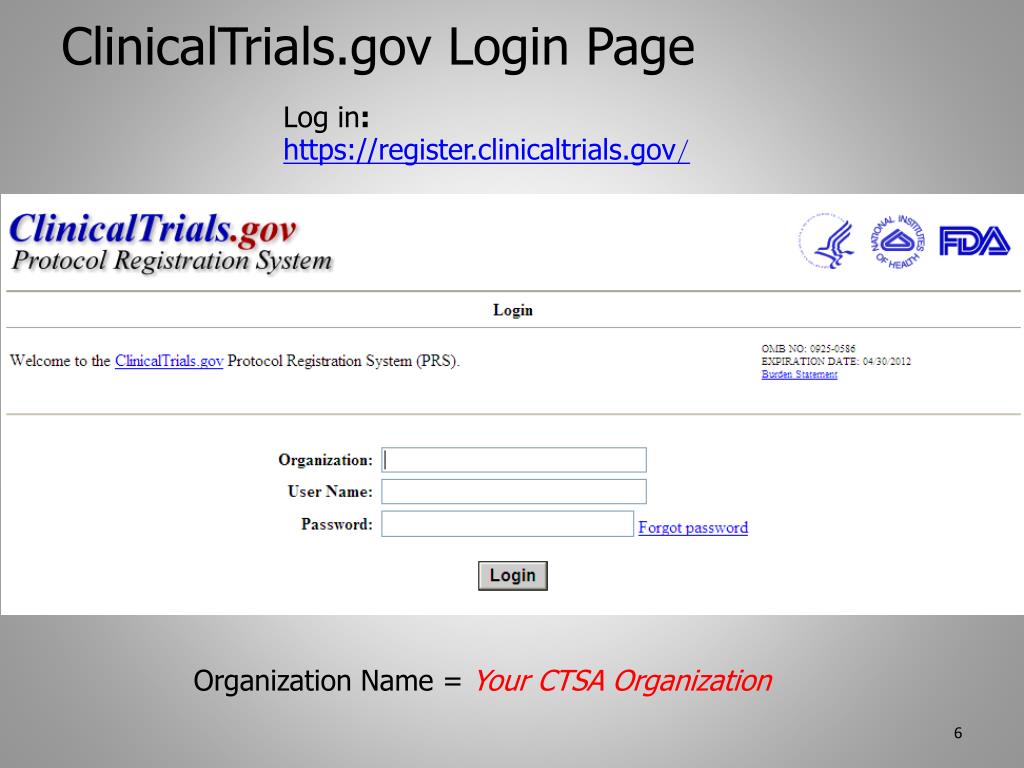
Login With Your Institutional Email.
Related Post: